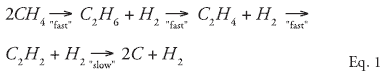The new process avoids the expensive syngas step in Fischer-Tropsch conversion, making it profitable even for small gas volumes.
Kenneth R. Hall, Texas A&M University; and Ben R. Weber, Jr., Synfuels International Inc., Dallas
Natural gas is a clean-burning and abundant energy resource, but much of it resides in locations remote from markets without an economic means of transport. One solution is to liquefy the gas, but this option involves considerable costs because it requires very low temperatures, making it practical only for large gas fields. Another solution is to convert the gas into hydrocarbon liquids using chemical processing. Gas-To-Liquids (GTL) technology using the Fischer-Tropsch process has been commercially available for more than 80 years to convert natural gas into “syngas” (a mixture of carbon monoxide and hydrogen) followed by conversion into liquid fuels. Unfortunately, Fischer-Tropsch technology is also expensive and requires large gas fields to justify the capital investment.
A Texas A&M University-led team has conceived a new process for converting natural gas into hydrocarbon liquids. The technology was enhanced by Synfuels International Inc., which licensed it in 1998. It is a “direct” conversion method that does not require producing syngas. The process is relatively simple, consisting of three reaction steps to produce liquids and two reaction steps to produce ethylene.
The GTL process can be used anywhere natural gas exists, from offshore platforms to remote onshore sites. This technology offers an alternative to flaring or venting natural gas when pipelines do not exist, are impractical, or are uneconomic. The liquids can be transported by liquid pipelines or in trucks or tankers. Thus, the process offers the opportunity to monetize a resource as well as to reduce undesirable emissions. The GTE technology is viable for locations near existing chemical industry infrastructure that requires ethylene and/or hydrogen.
INTRODUCTION
Natural gas often has been, and in some cases still is, a nuisance in petroleum production. Natural gas associated with a petroleum reservoir can present a serious disposal problem. In many locations, it is no longer acceptable to vent or flare the gas, and the lack of an appropriate gas-handling option can cause a shutdown in oil production.
On the other hand, gas is becoming a highly desirable commodity. As a fuel, it burns relatively cleanly, and any sulfur it may contain is easy to remove. As a chemical feedstock, it yields ethylene and propylene from its ethane and propane content, to form the basis of a massive chemical industry. Large reserves exist in many regions of the world, and it is likely that even greater reserves are available because major efforts to explore for gas are a relatively recent undertaking.
However, gas has a major drawback in transportation. Much of the gas appears in regions that are remote from markets or pipelines, and is commonly referred to as “remote” or “stranded” gas. Moreover, the gas is at a low density, requiring expensive large-diameter and high-pressure pipeline construction. This difficulty has been behind the recent drive to develop improved liquefaction technologies, compression technologies and GTL.
Another potential technology that has recently generated interest is a means to “activate” the methane in natural gas. Essentially, the hope for activation lies in finding a catalyst that can convert relatively inactive methane into an active compound, probably an olefin. For example, producing ethylene from methane in addition to or instead of ethane would greatly increase the value of the available feedstock. Producing hydrogen along with the ethylene would also benefit the chemical industry.
Texas A&M University researchers and Synfuels International have developed a new process that can convert natural gas economically into hydrocarbon liquids or into ethylene and hydrogen. Of course, Fischer-Tropsch (FT) technology has existed for nearly a century, and it can convert natural gas into hydrocarbon liquids. However, FT requires converting the gas first into syngas (a mixture of CO and H2), which is expensive. In addition, products of the FT process have an extremely broad, nearly normal distribution, containing everything from light gases to wax. The new process avoids the syngas step, and it has an asymmetric product distribution that truncates the production of heavy ends.
Economics dictates that FT plants be large, with capacities greater than 500 MMcfd, and preferably on the order of 1,000 MMcfd. However, the new process can be profitable even at a capacity of less than 10 MMcfd. Plants with capacities less than 20 MMcfd can be skid mounted, while those with capacities less than 50 MMcfd can be barge mounted. No technical upper limit exists for the size of the new plants, but economics can eventually make the capital investment prohibitive, as is presently the case with 1,000-MMcfd FT plants.
THE PROCESS
While methane activation is a topic of considerable interest in the petrochemical and energy industries, it also is difficult to achieve. However, by subjecting methane to temperatures in the range of 4,000°F, it is possible to convert methane into acetylene by the overall reaction:
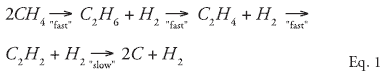
The “fast” and “slow” labels on the reactions are relative: The fast reactions occur in microseconds while the slow reaction occurs in milliseconds. Although acetylene is a very reactive molecule, it is unusual among hydrocarbons in that it is stable at these high temperatures. With careful attention to residence time (e.g., < 1 ms), it is possible to design a reactor that produces acetylene and hydrogen with very little carbon or coke formation. After hydrogenation, the entire realm of ethylene chemistry becomes available.
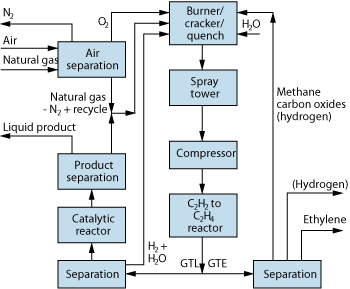
Figure 1 presents a schematic diagram of a possible arrangement for the process(es). Sweet natural gas is assumed to enter the process. An amine unit installed upstream of the process can remove H2S and CO2 effectively from the feed gas if necessary. It is also a good practice to run the inlet gas through a unit to remove the Liquefied Petroleum Gas (LPG) fraction because it does not make sense to pass the gas through an expensive GTL process to convert LPG into liquids. The LPG fraction is cut at the butane level to reduce expense.
 |
|
Fig. 1. Schematic diagram of a possible arrangement for the new gas-to-liquids/gas-to-ethylene process(es).
|
|
Commercial-grade oxygen is used in the combustion. The combustion products reach temperatures sufficient to convert much of the methane, and all other hydrocarbons in the gas, into hydrogen and acetylene as in Eq. 1. The design of the converter allows the feed gas to mix with the combustion products and then enter a quench zone in which water is sprayed into the stream. The quench can be performed easily within 1 ms of introducing the feed gas into the combustion products. Depending on the size of the converter (larger is better), it is possible to convert 50-65% of the methane and all of the other hydrocarbons in the feed gas into acetylene and hydrogen. Of course, subjecting hydrocarbons to these temperatures causes coke formation in the cracker. Some steam is added in the combustion zone to reduce this effect and to regulate the combustion products’ temperature. This ensures that overall coke formation is very low, less than 1% of the inlet carbon. The gas stream exiting the converter consists of acetylene, hydrogen, unconverted methane, carbon oxides and nitrogen and/or nitrogen oxides.
The gas stream is then washed (to remove carbon granules/particles and any green oil formed in the converter) and cooled to ambient temperature before it is compressed to hydrogenation pressure (to 400 psia from 100 psia). At this point, the acetylene is catalytically hydrogenated to ethylene using some of the hydrogen produced in the cracker. It is possible to perform this hydrogenation in the gas phase or in a liquid phase. The gas-phase reaction is a common operation, but the liquid-phase hydrogenation is a proprietary process invented by Synfuels International. At this point, the user must decide if ethylene (and possibly hydrogen) is desired as a product or liquid.
Assuming the GTE path is selected, the stream is run into a cryogenic separation unit that splits out the ethylene and hydrogen (if desired). The remaining components of the stream are methane, carbon monoxide, possibly hydrogen, and nitrogen compounds. The nitrogen compounds are removed, and the remainder flows back to the cracker as fuel. The ethylene and hydrogen can feed one or more chemical processes. This option assumes proximity of a chemical industry that can accept the ethylene and hydrogen. Although these products are generally more profitable than liquid fuels, transporting them over long distances is even more difficult than transporting natural gas. The hydrogen is less dense than natural gas (requiring large and high-pressure pipelines) and ethylene is reactive and likely to convert into other compounds in transit.
Assuming the GTL path is chosen, the stream passes to a catalytic reactor in which the ethylene, and possibly a small amount of the methane, is oligomerized into a hydrocarbon stream that passes to a stabilizer. This unit produces the liquid product and a recycle stream of light hydrocarbons (C5 and lighter) for the thermal converter. This product results when the reactor operates at low (near atmospheric) pressure. High-pressure operation would result in a jet fuel, while yet-higher pressures would produce a diesel fuel. It is possible to operate the oligomerization reactor in a manner to reduce the aromatic content of the liquid product. This operation might be preferable for gasoline production, but jet fuel requires significant aromatic content. The product that results after stabilizing the stream is a 95-octane, C8-average “fuel.” It is about 30% aromatics (but low in benzene), 40% isoalkanes and 30% cycloalkanes. Alternate operation could reduce the amount of aromatics, but the relative amounts of normal, iso- and cycloalkanes would remain about the same. Rather than using the product as a fuel, a higher-profit use could be to separate the aromatics from the alkanes. The (liquid and therefore transportable) aromatics would be a good feedstock for a chemical product, while the alkanes would be a good alkylation stock for gasoline refineries.
CHEMISTRY
While the actual kinetic mechanism is undoubtedly complex, the overall effect of the converter is Eq. 1. The reaction is quenched before the final step, resulting in the overall reaction:

Of course, some carbon and granular (not sticky) black coke would form, but the amount is very small. Equation 2 suggests another modification for the process. Natural gas contains a significant amount of C2+ compounds. These compounds are relatively easy to convert (crack) into acetylene. However, they remove energy from the converter that could have converted the methane into acetylene. Adding a demethanizer to the inlet stream would permit the converter to operate on nearly pure methane; thus all of the energy would be available for its conversion. Then a smaller cracker could accommodate the C2+ stream at much lower temperatures. The overall effect should be to increase the amount of carbon entering the process that leaves as acetylene, and eventually as product. The demethanizer need not be too selective between methane and ethane. Methane contamination in the ethane would not provide very different results from those presented here. A smaller amount of ethane in the methane would improve the methane conversion.
CONCLUSIONS
The process described above is not FT technology. It does not seek to produce syngas, it does not use FT catalysts, and the product distribution is not at all similar to that of FT. If the desired product is gasoline or jet fuel or diesel, the new process produces relatively more than FT because it does not produce the long tail of heavy (and undesirable) products. In fact, the new process is actually more nearly a methane activation process: it converts the methane (and all other hydrocarbons) in the natural gas into acetylene, a reactive compound.
Because the new process is simpler than FT, it is less expensive and can operate with much smaller flows while remaining economic. As a result, this process opens options not available to FT processes. It is possible to reduce gas flaring because, if about 10 MMcfd of flare gas is available, it can be converted economically into liquid fuel or ethylene/hydrogen. Relatively small gas fields may become attractive for development because small plants can be portable. When one field depletes, the plant can be moved to another location. The portability also brings really remote fields into play, such as in jungles or on isolated islands. The process can accept relatively small amounts of associated gas from oil reservoirs and remove the threat of closure due to regulations restricting flaring, venting or reinjection. It would also be possible to address the potential development of low-value coal-seam gas; in fact, this methane-rich gas would be an ideal feedstock for the process. Finally, no technical obstacle exists for building a plant of any size larger than 10 MMcfd.
Synfuels International is working to commercialize the process worldwide. The company has constructed a pilot/demonstration plant that can process up to 50 Mcfd, Fig. 2.
 |
|
Fig. 2. Synfuels International has constructed a pilot/demonstration plant near Texas A&M University to produce gasoline from natural gas. The plant can process up to 50 Mcfd.
|
|
In November 2007, Synfuels International agreed to sell 15% of the company to Aref Energy Holding, a company listed on the Kuwaiti stock exchange. The partnership will provide the funding and expertise to market and license the technology on a worldwide basis in the abundant gas territories of the Middle East and North Africa. The deal includes plans to build the first commercial plant based on the technology. 
BIBLIOGRAPHY
Hall, K. R., “A new route from natural gas to liquid fuels,” Chemical Engineering, June 2001.Hall, K. R., “A radically new process to convert natural gas into hydrocarbon liquids,” presented at the AIChE National Meeting, Houston, 2001.
Hall, K. R., “New GTL method for fewer heavy fractions,” Hydrocarbon Processing, November 1, 2002.
Hall, K. R., “New low-cost GTL naphtha scheme debuts,” GTL News, August 2000.
Peterson, E. et al., “The ECLAIRS process for converting natural gas to hydrocarbon liquids,” presented at the 4th GTL Technology and Commercialization Conference, Doha, 2004.
“A new gas to liquids (GTL) or gas to ethylene (GTE) technology,” Catalysis Today, 106, 2005, pp. 243246.
|
THE AUTHORS
|
|
|
Kenneth R. Hall, the Jack E. and Frances Brown Chair and professor of chemical engineering at Texas A&M University, has been the department head of chemical engineering; associate dean of engineering; assistant, associate and deputy director of the Texas Engineering Experiment Station and associate vice chancellor for engineering. He has directed the Chemical and Transport Systems division of the National Science Foundation. He has 226 refereed papers, seven books and 12 patents.
|
|
|
|
Ben Weber is the founder, president, CEO and sole director of Weber Energy Corp. A native of Dallas and graduate of the University of Texas at Austin, he is an oil and gas operator. Mr. Weber was responsible for incorporating Synfuels International Inc. to expand the R&D and deploy the technology worldwide.
|
|
|