Rice University engineers advance NMR models for oil and gas
HOUSTON -- New work by Rice University chemical engineers may make it easier to characterize unconventional oil or gas shale formations.
Nuclear magnetic resonance (NMR) tools are common in the oil industry to find rich veins of crude oil and methane for extraction, particularly through hydraulic fracturing of deep shale deposits. The Rice team of professors George Hirasaki and Walter Chapman and research scientists Philip Singer and Dilip Asthagiri have refined their previously reported techniques to characterize methane over a wide range of densities and temperatures spanning the liquid state to the gas state.
The researchers have tuned their molecular dynamics models to better distinguish between molecules with either spin-lattice relaxation or spin-spin relaxation, which will improve the ability of NMR to differentiate oil and gas from water and measure the amount present in the rock, even in tight pore formations.
The Journal of Chemical Physics has published their paper as an open-access "editor's pick."
NMR tools inserted into a wellbore characterize the formations they see at various depths as they look for the right spots to initiate horizontal drilling and fracing, which pump water, proppants and thickening agents downhole under pressure to fracture shale and free the hydrocarbons.
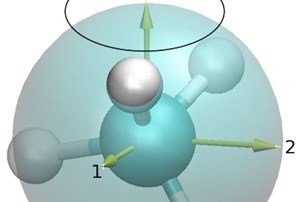
The tools use the same principle that magnetic resonance imagers employ to see inside the human body: They orient and manipulate the nuclear magnetic moments of hydrogen atoms – like the invisible hands of a compass – using a combination of static and pulsed radio-frequency magnetic fields.
After the pulsed field is turned off, these moments take a few seconds to "relax" back to their original orientations. NMR devices detect that relaxation. Because relaxation times differ depending on the molecule and its environment, NMR can help identify whether a molecule is gas, oil or water as well as gather information about the size of the pores that contain them.
"NMR relaxation times and viscosity are inversely correlated for light crude oils," Singer said. "However, when you dissolve methane in crude oil, that correlation changes because methane has a different mode of relaxation than all the other (hydrocarbon) alkanes.
"The reason for that is because methane is a spherically symmetric molecule compared with other alkanes, which are linear, long and branched. Therefore, methane has a completely different geometry resulting in a different relaxation mode, both in bulk and in tight pores."
Identifying that "different" mode was critical, Chapman said. "Methane mixed with hydrocarbons affects viscosity in interesting ways," he said. "We need to understand, essentially, how much methane is in the system to relate to that response.
"What we can do in the laboratory is measure fluid properties, and we can measure NMR response," he said. "We like to relate the two in as rigorous a theoretical approach as we can so that when we have a tool in the wellbore and it's measuring properties of fluid in the reservoir, we can interpret those results accurately."
The researchers said the interpretation of the NMR signal varies from one field – or even one well – to the next, which complicates calculations for producers characterizing a reservoir. "The kind of studies we are doing can become a bridge to interpreting those signals," Asthagiri said.
"We hope to have a model that describes all the physics so that we don't have to shift (the model) from one well to a different well," Chapman added. "One size will fit all. If we can include all the physics, the model will be representative of the true physics in the reservoir."
"Potentially we can create a library of relaxation behavior in the lab for different types of confinement, for small pores and for different chemistries of pores," Asthagiri said. "Then we can look at a signal and read what material we are looking at downhole."
"As we study fluids in porous media, we're going to find that confinement changes the relaxation behavior, resulting in a different NMR response," Chapman said. "Understanding the physics behind this response is important in characterizing the shale."
Chapman is the William W. Akers Professor of Chemical and Biomolecular Engineering and associate dean for energy research in the George R. Brown School of Engineering. Hirasaki is the A.J. Hartsook Professor Emeritus of Chemical and Biomolecular Engineering.
The Rice University Consortium on Processes in Porous Media and the American Chemical Society Petroleum Research Fund supported the research, with computing resources supplied by the National Energy Research Scientific Computing Center, which is supported by the Office of Science of the U.S. Department of Energy, and the Texas Advanced Computing Center at the University of Texas at Austin.